Is there a vaccine for the coronavirus?
The answer is yes and no. Multiple vaccines are currently in different phases of testing, but none has been approved for use with the public.
How do vaccines work?
The immune system has two branches: the innate immune system and the adaptive immune system. The innate system responds rapidly but does not recognize specific pathogens such as the coronavirus. When the immune system encounters a pathogen for the first time, it takes about two weeks for the adaptive immune system to mount a specific response. This may be too long, and the infected individual may become very sick or die.
A vaccine displays parts of the virus or bacterium to the immune system. This effectively “tricks” the immune system into reacting as if it has seen the pathogen before. When it sees the real pathogen, it mounts a very rapid immune response and protects the vaccinated individual.
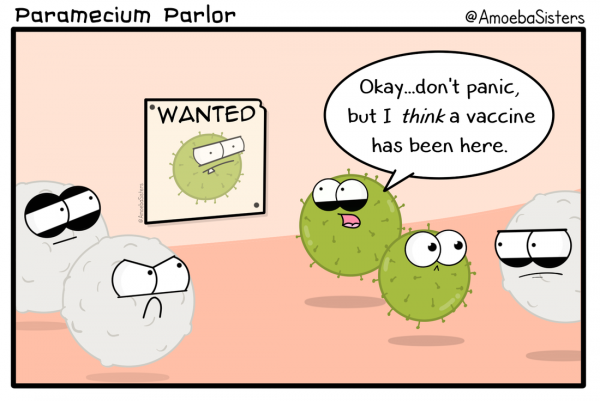
Picture source: Paramecium Parlor by the Amoeba Sisters
What is herd immunity?
When enough people in a community are immune to a specific disease, it can no longer spread very quickly in the population. This hampered spread is because most of the people who come into contact with the patient are not susceptible to the infection and thus cannot give it to others. The best way to achieve herd immunity is through vaccination.
How many coronavirus vaccines are being developed right now?
The number is changing rapidly every day. As of April 9, there are 115 vaccine candidates, with 73 of them at exploratory or preclinical stages. Five of the candidates are approved for clinical trials, which means they will be tested in human subjects.
Which ones are the most promising?
It is too early to say which vaccine candidates would prove to be effective; however, the five that are in clinical trials are further along in the process of vaccine development. They are mRNA-1273 from Moderna in conjunction with the National Institute of Allergy and Infectious Diseases, Ad5-nCoV from CanSino Biologicals in conjunction with the Institute of Biotechnology of the Chinese Academy of Military Medical Sciences, INO-4800 from Inovio, LV-SMENP-DC and pathogen-specific aAPC from Shenzhen Geno-Immune Medical Institute. The clinical trials for INO-4800 will be a collaboration between the U.S., China and South Korea.
What makes each of these vaccine candidates different from the others?
There are different methods to present the features of a virus to the immune system. Moreover, for each method, one can choose different parts of the virus to show the immune system. There is no clear answer, at this moment, to which of these methods are better, or which part of the virus should be shown to the immune system. Clinical trials will demonstrate which vaccines are effective.
When can we expect a vaccine?
Historically, a vaccine’s development process takes close to 11 years. The current record of the fastest vaccine development (for a vaccine against Ebola) took five years. Due to the severity of COVID-19, countries are investing many resources, hoping to shorten the process.
The U.S. Department of Health & Human Services aims to roll out a vaccine in early 2021. This timeline, however, is dependent on the clinical trial results. Most experts forecast that this process will take at least 12–18 months. The WHO is also working on making sure that everyone who needs a vaccine could have access to one and that no country hoards them.
Why does it take so long to develop a vaccine?
According to the CDC, there are multiple stages that a vaccine candidate must pass in order to receive approval for use in the general public. These include the exploratory stage, preclinical stage, clinical development, regulatory review and approval, manufacturing and quality control.
Clinical development can be further divided into three phases. In Phase I, only a small number of human subjects is tested with the vaccine candidate. In Phase II, the test group is expanded to people who would represent the intended patient demographics. In Phase III, the vaccine would be given to a large number of volunteers to ensure efficacy and safety. Vaccine candidates to be approved for usage need to go through this extensive vetting process.
Would they be safe and effective?
Rigorous clinical trials will ensure that an approved vaccine will be safe and effective. The technologies being used to produce these vaccines are already in use for other approved vaccines.
What organizations are working on vaccines?
According to Le et al. on Nature Reviews, 72% of the confirmed vaccine candidates are developed by private companies, with 28% from academic, public and non-profit institutes. Among the confirmed vaccine candidates, some 46% of the developers are from North America, while 18% are in China, another 18% in the rest of Asia Pacific, and 18% in Europe.